|
The Periodic Table
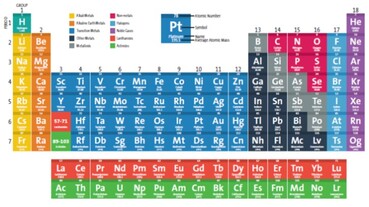
Click on the picture above for a printable copy of the Periodic Table of Elements.
The Periodic Table
The periodic table of the elements was first introduced in the mid-19th century by Dmitry Mendeleev. He organized the elements by atomic number, which is equal to the number of protons found in the nucleus of the element’s atoms. Learn Learn more about the periodic table with this interactive table.. (Click on the Picture Below) And What the Heck is Polonium?
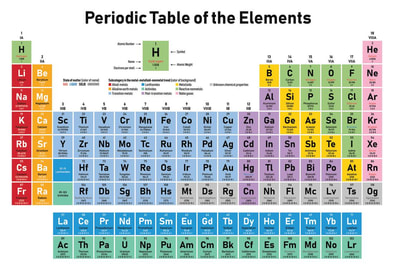
Different elements have different properties from Iron to the Noble Gases. This interactive periodic table provides "did you know?" facts for all the elements. A great place to do research! (Click on the Picture Below) |
Mystery Element
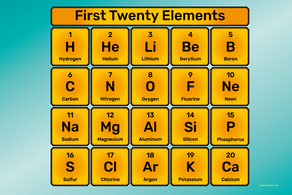
Explore this interactive periodic table by clicking on the elements to find out such things as name, atomic mass, and classification. Then quiz yourself with an interactive game to place a dozen elements onto a blank table. (Click on the Picture Below) The Noble Gases
The noble gas configuration of electrons makes these atoms chemically inactive, zero action, so they tend to be on their own and unnoticed. This stop of chemical activity means complete rest, an inert state like a lazy Sunday afternoon in a rocking chair. Learn all about the Noble gases right here! Take the quick quiz afterwards (it's below). (Click on the Picture Below) Take the quick quiz here
|